Modern steelmaking processes, particularly electric arc furnace (EAF) and basic oxygen furnace (BOF) operations, produce significant quantities of zinc-containing steel dust. These residues, often captured through bag filters, present serious challenges for recycling due to the presence of zinc and other volatile heavy metals. Improper handling of such waste not only leads to environmental hazards but also disrupts the sintering and blast furnace processes in steel production. Fortunately, Rotary Hearth Furnace (RHF) technology, combined with cold briquetting techniques, offers a proven solution for dezincification and metallization, transforming hazardous waste into valuable raw materials.

Rotary Heath Furnace
What is Zinc-Containing Steel Dust
Steel dust generated during smelting processes typically contains a mixture of iron (Fe), zinc (Zn), lead (Pb), chlorine (Cl), alkali metals, and carbon (C). These materials originate from a variety of sources, such as galvanized scrap, coatings, and fluxing agents. Among them, zinc is particularly problematic due to its low boiling point, causing it to vaporize during smelting and re-condense within the flue gas system.
What is RHF Technology
https://www.youtube.com/embed/eqlZq3r6BAA
Rotary Hearth Furnace (RHF) is a direct reduction method ideal for processing fine-grained waste materials like 钢厂除尘灰. It operates in a reducing atmosphere at temperatures of 1200–1250°C, converting iron oxides into metallized iron and volatilizing zinc for recovery.
RHF technology is particularly suited for dezincification due to the following reasons:
- High efficiency in removing volatile metals such as zinc and lead.
- Short residence time, allowing for continuous and fast processing.
- Energy recovery potential through off-gas handling.
- Compatibility with cold briquettes, improving material handling and stability.
The RHF method is increasingly being adopted by steel plants worldwide as a cleaner, more sustainable approach to treating dust with complex compositions.
What Are the Raw Materials for RHF Dezincification?
| Sources | Fe | Zn | Other Content |
| BF Dust | 30-50% | 1-5% | 10-30% |
| BOF Dust | 50-70% | 0.5-3% | High in CaO/MagO |
| EAF Dust | 20-40% | 10-25% | Pb/Cd |
Typical Feed Composition:
- Zinc-Containing Dust: 70–85%
- Carbon as Carbonaceous Reductant: 10–20%
- Binder: 1–3%
The Process of RHF Dezincification
The typical dezincification process using RHF consists of several key stages:
Step 1: Raw Material Pretreatment
Drying and Screening: Removes moisture in the dust and grind the dust into appropriate particles.
Chemical analysis: Assesses Zn, Fe, C, etc. content to guide process parameters
Carbon Source Selection:
- Internal Carbon: Residual carbon in dust (e.g. BF dust contains 10-30% C.)
- External Carbon: Anthracite coal 或者 能够生成 (Fixed Carbon ≥75%, Volatile Matter ≤10%).
Step 2: Mixing
In this step, mixing dust with 粘合剂 、 other agents such as carbon.
- Optimize Carbon/Oxygen (C/O) ratio to 1.0–1.2: if <1.0, insufficient reduction, low dezincification rate; if >1.2, increase carbon residual in the finished product and influence the effects of briquettes in steelmaking.
- Maintain Zn content ≤8% in the mixture.
- Adjust TFe ≥45%; add mill scale if needed.
- Target alkalinity: 0.18–1.2% (if the alkalinity of mixture >1.5%, might stick to the furnace under high temperature).
Step 3: Agglomeration (压块)
Fine particles are shaped into briquettes using high-performance binders, making them suitable for furnace feeding.
Briquetting Machine:
- Double-Roller Briquette Machine: the size of briquettes made by this machine ranges from 20-30mm.
- Disk Granulator: the size of briquettes ranges from 10-20mm.
Step 4: Drying

Drying Machine
Dry the briquettes to remove the moisture in briquettes (moisture ≤2%)
Drying machine: Travelling Grate and Belt Dryer
Drying Temperature: 200-300℃; Drying time: 30min
Step 5: Reduction in Rotary Hearth Furnace

Inside the RHF
Feed the briquettes into RHF. In the RHF, there are several zones: feeding zone, preheating zone,reduction zone 1, reduction zone 2, and reduction zone 3, and discharge zone.
- Preheating Zone: ~950–1100°C
- Reduction Zones (3 stages): ~1200–1250°C
- The total time of reduction: 20-30mins
Inside RHF, there are several reductions are happening:
Iron Recovery (Metallization):
Fe₂O₃ + C → Fe + CO₂
At around 1250°C, carbon particles react with iron oxides to produce metallized iron. In carbon-containing briquettes, carbon and iron oxides are uniformly distributed throughout the briquette. Once the briquette reaches the required temperature, numerous carbon particles within the briquette react with iron oxides, enabling internal reduction throughout the entire structure. This process is referred to as “self-reduction.”
The resulting metallized iron remains in the solid state and can be recycled into the steelmaking process.
Zinc Recovery (Dezincification):
ZnO + C → Zn↑ + CO↑
Zinc enters the rotary hearth furnace in the form of ZnO. As the furnace rotates, the temperature gradually increases. When it reaches around 1000°C or higher, a reduction reaction occurs: ZnO is reduced to gaseous zinc (Zn↑), which then migrates with the gas flow. In the presence of residual oxygen in the flue gas, the zinc vapor is re-oxidized into ZnO in the gas phase. These ZnO particles are then captured and condensed downstream, forming zinc-rich dust suitable for further refining.
Therefore, the behavior of zinc in the rotary hearth furnace is essentially a high-temperature migration and transformation process of zinc oxide.
Step 6: Off-Gas Treatment
Off-gas contains Zn and Pb vapor.
They are condensed in a cooling system and collected as zinc-rich dust
Step 7: Metallized Product Handling

The final products (DRI) contain ~85–90% metallized iron and can be fed back into blast furnace or EAFs.
Key Factors in RHF Dezincification You Need to Know
Pulverization Rate
Definition: Pulverization rate refers to the proportion of pellets/briquettes that pulverize/break into fine powder (<1mm) inside the Rotary Hearth Furnace (RHF) due to mechanical impact, thermal stress, or chemical reactions.
Why Does It Matter?: A high pulverization rate (e.g., >20%) can lead to decreased furnace permeability, lower metallization efficiency, and difficulty in zinc recovery.
Factors That Influence Pulverization Rate:
- Mechanical strength: Low cold/hot compressive strength (e.g., from disc pelletizing) makes pellets prone to breakage.
- Thermal stress: Rapid heating or cooling and temperature gradients can cause crack propagation.
- Binder failure: Decomposition of binders at high temperatures can result in structural collapse
Negative Impacts of High Pulverization Rate on Dezincification Process:
- Lower zinc recovery rate: Pulverized fine particles are carried away by flue gas and are difficult to recover via condensation.
- Increased energy consumption: Accumulated fine powder hinders heat transfer, requiring longer reduction time or higher furnace temperatures.
- Reduced metallization efficiency due to lower furnace permeability.
Metallization Rate
Definition: Metallization rate refers to the proportion of iron oxides in the agglomerates that are reduced to metallic iron (Fe), indicating the degree of completion of the reduction reaction.
- Qualified Metallization Rate: ≥85%
Factors That Influence Metallization Rate:
- Reduction Temperature and Time: High temperatures (1250–1350°C) and sufficient residence time help promote the complete reduction of iron oxides.
- Reducing Atmosphere: A high concentration of CO or H₂ (e.g., CO ≥ 20%) accelerates the reduction reactions.
- Pellet Porosity: Pellets with appropriate porosity (e.g., produced by roller press briquetting) allow better gas diffusion and uniform reaction.
- Carbon-to-Iron Ratio (C/Fe): An adequate amount of carbonaceous reductant (C/Fe ≈ 0.8–1.2) is essential for driving the reduction process.
Dezincification Rate
Definition: Dezincification rate refers to the proportion of zinc (present as ZnO in the pellets) that is reduced to metallic zinc vapor (Zn) and removed during processing.
- Qualified Dezincification Rate:
Steel plants require a dezincification rate of ≥90% to meet the zinc content standards for returning dust to the blast furnace or BOF (Zn < 1%).
Factors That Influence Dezincification Rate:
- Reduction Temperature and Time:
ZnO reduction occurs within 900–1100°C. Excessively high temperatures (>1200°C) may cause zinc vapor to re-oxidize.
- Furnace Atmosphere:
A strongly reducing atmosphere (high CO concentration) promotes ZnO reduction. However, excessive CO₂ can oxidize zinc vapor (Zn + CO₂ → ZnO + CO).
- Pellet Pore Structure:
High-porosity pellets (e.g., from disc pelletizing) facilitate the rapid escape of zinc vapor, but may compromise mechanical strength.
- Zinc Speciation:
Zinc in the form of free ZnO is easier to remove. When zinc is bound in compounds like zinc ferrite (ZnFe₂O₄), higher temperatures and stronger reducing conditions are required.
Dezincification Rate vs. Metallization Rate vs. Pulverization Rate
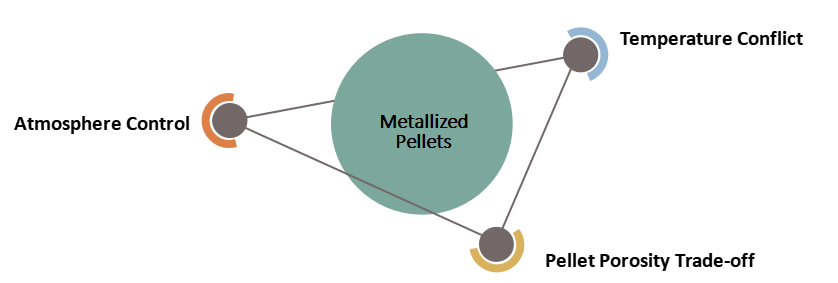
Dezincification rate, metallization rate, and pulverization rate are three key indicators to monitor in RHF processing. However, optimizing one of these indicators may hinder the improvement of the others. The conflicts among these three indicators are explained below
1. Temperature Conflict:
The optimal temperature for dezincification (900–1100°C) is lower than that required for deep reduction of iron oxides (>1200°C). A segmented temperature control strategy is needed to balance both.
High temperatures favor metallization but may suppress zinc removal by causing zinc vapor re-oxidation or iron sintering.
2. Atmosphere Control:
A strongly reducing atmosphere benefits dezincification but may cause metallic iron sintering, reducing metallization reactivity.
3. Pellet Porosity Trade-off:
High-porosity pellets aid dezincification but increase the risk of pulverization, which reduces furnace permeability and metallization rate.
Solutions:
Use Roller Press Briquette Machine with Porosity Control:
Design pellets with a gradient structure—dense outer shell and porous core—to balance zinc vapor release and mechanical strength. (Target porosity: 10–15% to improve briquette integrity.)
Two-Stage Heating Process:
- Apply “Low-Temperature Dezincification + High-Temperature Metallization” approach:
- Preheating zone: 950–1100°C for zinc removal
- High-temperature zone: Hold at 1250°C for iron reduction
Use of High-Temperature Resistant Binders:
- Select binders that maintain pellet thermal stability during high-temperature operations.
Other Challenges in Cold Briquetting of Zinc-Containing Dust in Rotary Hearth Furnace
High Pulverization Rate of Pellets:
Pellets are prone to breakage and disintegration under thermal and mechanical stress, leading to high pulverization rates during handling or heating.
Presence of Undigested Alkaline Oxides (e.g., CaO, MgO):
- Pellets made from materials containing free CaO (f-CaO) or MgO are prone to disintegration and pulverization.
- f-CaO can absorb moisture and expand by 1.5–2.5 times, causing the pellets to crack and disintegrate during drying or storage.
Fine Particle Size of Solid Waste Powder:
Materials such as zinc-containing dust and carbon powder often have very fine particles and high carbon content, making them difficult to form into pellets.
Importance of Binder Selection
Compatibility Analysis:
Raw Material Formulation and Binder Adaptability:
1. The binder must be compatible with various types of zinc-containing dust, particularly those with high alkalinity, and should not react adversely with CaO, MgO, etc.
2. For high-alkaline dusts, customized binder formulations or alkali-resistant binders should be developed to ensure strength and stability.
3. Chemical stability of the binder is crucial, as it directly affects the performance of the pellet during high-temperature reduction.
Pellet Strength and Thermal Stability:
1. The binder should ensure green pellet compressive strength ≥10 N/pellet, drop resistance >3 times from 0.5 m, and dried strength >700 N. This helps avoid breakage during transport.
2. Under high temperatures, the binder must maintain integrity to prevent pulverization. Organic–inorganic composite binders and high-temp resistant additives can enhance thermal stability.
3. Both cold and hot strength properties of the binder significantly affect pellet quality and must be precisely controlled to ensure smooth processing.
Reduction Efficiency and Zinc Removal Rate:
1. Binder dosage must be carefully controlled to avoid blocking gas diffusion and reducing pellet permeability, which would lower reduction efficiency.
2. Optimizing pellet porosity enhances gas flow and promotes zinc vapor removal, supporting efficient operation.
3. The binder’s effect on permeability is closely related to reduction and dezincification efficiency and must be finely tuned.
Advantages of Jianjie Binder

Jianjie Binder For RHF Dezincification
High-Temperature Resistance:
- Jianjie binder for RHF dezincification contains high-temperature resistant components that interact with raw materials to enhance thermal stress resistance.
- Maintains binding strength at temperatures above 1000°C, reducing pulverization rate and meeting the dezincification requirements of rotary hearth furnaces.
Strong Adhesion:
- Formulated with high-bond-strength components, allowing for low addition rates while ensuring excellent adhesion.
- Results in high pellet forming rate, strong green pellet drop resistance, and high cold strength.
Resistance to Free CaO:
- Specially formulated to resist the effects of residual f-CaO, preventing pellet cracking, disintegration, and pulverization caused by hydration and expansion.
The reuse of steel mill dust through RHF technology presents a promising solution for sustainable resource recovery. However, achieving the optimal balance between dezincification rate, metallization rate, and pulverization rate remains a technical challenge. Jianjie’s advanced binder solutions and cold briquetting technologies help industrial users overcome these conflicts, maximizing economic value while reducing environmental impact.
Looking to improve your RHF performance and resource recovery efficiency? Contact Jianjie today to learn more about our customized binder systems and full-process support for industrial agglomeration.
FAQ:
Q1: Why is zinc-containing steel dust problematic for steelmaking?
A: Zinc interferes with sintering and blast furnace operations due to its volatility and reactivity. When vaporized during high-temperature processing, zinc can re-condense and cause operational disruptions. It must be removed—through a process like RHF dezincification—before recycling the dust back into steel production.
Q2: What’s the ideal temperature range for zinc removal vs. iron reduction?
A: Zinc removal (dezincification) occurs optimally between 900–1100°C. Metallization of iron oxides requires higher temperatures, typically 1200–1250°C. Therefore, a segmented or two-stage heating strategy is used: low-temperature preheating for zinc removal, followed by high-temperature zones for iron metallization.
Q6: Why do some briquettes pulverize more easily than others?
A: Factors include weak mechanical strength, thermal expansion, and binder degradation at high temperatures. Briquettes made with poor-quality binders or from dusts high in free CaO/MgO are especially prone to pulverization. Optimizing binder selection and briquette porosity helps improve resistance to breakage.